Molecular Orbital Theory (MO Theory) is a fundamental concept in quantum chemistry, providing insights into the electronic structure and bonding of molecules. Developed by Linus Pauling and Robert Mulliken in the 1930s, MO Theory extends the ideas of atomic orbitals to molecules, emphasizing the formation of molecular orbitals through linear combinations of atomic orbitals. These molecular orbitals, representing the spatial distribution of electrons, play a crucial role in understanding properties such as bond strength, stability, and electronic behavior in molecules. MO Theory has become an indispensable tool for predicting and explaining molecular properties, influencing diverse fields including chemistry, materials science, and biochemistry.
Understanding Electronic Structure and Bonding in Molecules
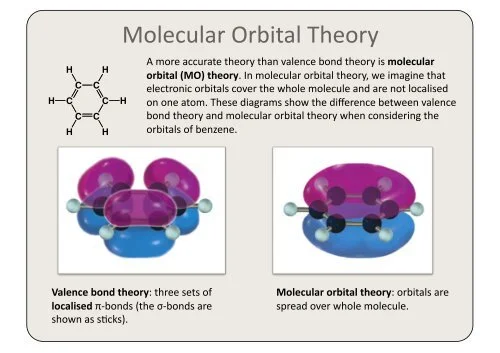
What Is Molecular Orbital Theory?
Molecular Orbital Theory (MO Theory) is a key concept in quantum chemistry that delves into the electronic structure and bonding of molecules. Introduced by scientists such as Linus Pauling and Robert Mulliken, MO Theory extends atomic orbital ideas to molecules. It emphasizes the formation of molecular orbitals through the linear combination of atomic orbitals, providing insights into electron distribution, bond strength, and molecular stability. By analyzing the spatial distribution of electrons in these molecular orbitals, MO Theory proves invaluable in explaining and predicting the properties of molecules, contributing significantly to our understanding of chemical bonding and reactivity.
Linear Combination of Atomic Orbitals
The Linear Combination of Atomic Orbitals (LCAO) is a fundamental concept in quantum chemistry and molecular orbital theory. It describes the formation of molecular orbitals (MOs) through the mathematical combination of atomic orbitals (AOs) from the constituent atoms of a molecule. By adding or subtracting these atomic orbitals with appropriate coefficients, new molecular orbitals are generated. The coefficients represent the contributions of each atomic orbital to the molecular orbital, influencing its shape, energy, and electron distribution. LCAO is a crucial aspect of understanding how atomic orbitals combine to form the overall electronic structure of molecules, providing insights into bonding and molecular properties.
Conditions for Linear Combination of Atomic Orbitals:
The Linear Combination of Atomic Orbitals (LCAO) method is used to construct molecular orbitals (MOs) from atomic orbitals (AOs). The conditions for applying LCAO in the formation of MOs include:
-
Same Symmetry: The atomic orbitals being combined to form a molecular orbital should have similar symmetry. This is essential for constructive interference and effective overlap between the orbitals.
-
Similar Energy: Atomic orbitals with comparable energy levels are more likely to form molecular orbitals with reasonable stability. This condition helps in the proper mixing of orbitals.
-
Appropriate Phasing: Phasing refers to the sign (positive or negative) of the atomic orbitals when they combine. Constructive interference occurs when orbitals of the same phase combine, while destructive interference occurs when orbitals of opposite phases combine.
-
Normalization: The resulting molecular orbitals should be normalized, meaning that the sum of the squares of the coefficients of the contributing atomic orbitals is equal to 1. This ensures that the probability of finding an electron is conserved.
-
Orbital Overlap: For effective LCAO, there must be significant overlap between the atomic orbitals. Stronger overlap leads to more stable molecular orbitals.
By satisfying these conditions, the LCAO method provides a powerful framework for understanding the formation of molecular orbitals and, consequently, the electronic structure of molecules.
What Are Molecular Orbitals?
Molecular orbitals (MOs) are mathematical functions that describe the distribution of electrons within a molecule. These orbitals result from the quantum mechanical combination of atomic orbitals (AOs) from the constituent atoms. Molecular orbital theory explains how electrons are distributed in a molecule and how the molecule behaves chemically.
There are two main types of molecular orbitals:
-
Bonding Molecular Orbitals (BMOs): These orbitals result from the constructive interference of atomic orbitals. Electrons in bonding molecular orbitals help hold the atoms together, contributing to the stability of the molecule. BMOs are lower in energy than the original atomic orbitals.
-
Antibonding Molecular Orbitals (ABOs): These orbitals result from the destructive interference of atomic orbitals. Electrons in antibonding molecular orbitals do not contribute to the stability of the molecule; in fact, they can destabilize it. Antibonding molecular orbitals are higher in energy than the original atomic orbitals.
The number of molecular orbitals formed is equal to the number of combining atomic orbitals. The molecular orbitals are filled with electrons according to the Aufbau principle, Pauli exclusion principle, and Hund’s rule, similar to how electrons fill atomic orbitals.
Understanding molecular orbitals is crucial for explaining the electronic structure, stability, and reactivity of molecules in various chemical processes. The combination of atomic orbitals to form molecular orbitals is often depicted using molecular orbital diagrams.

Download Chemistry Notes
Difference between Bonding and Antibonding Molecular Orbitals
Bonding and antibonding molecular orbitals (BMOs and ABOs) are two types of molecular orbitals formed through the combination of atomic orbitals in a molecule. Here are the key differences between them:
In molecular orbital theory, the interaction between atomic orbitals to form bonding and antibonding orbitals plays a crucial role in determining the electronic structure and properties of molecules. The filling of these orbitals with electrons follows the principles of quantum mechanics and influences the overall stability and reactivity of the molecule.
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What is Molecular Orbital Theory (MO Theory)?
Answer. Molecular Orbital Theory is a concept in quantum chemistry that explains how atomic orbitals combine to form molecular orbitals, influencing the electronic structure and bonding in molecules.
Q2. How are Molecular Orbitals formed?
Answer. Molecular Orbitals are formed through the linear combination of atomic orbitals (LCAO) from the constituent atoms in a molecule. The combination can be either constructive (forming bonding orbitals) or destructive (forming antibonding orbitals).
Q3. What is the difference between Bonding and Antibonding Molecular Orbitals?
Answer. Bonding Molecular Orbitals (BMOs) result from constructive interference and contribute to stability, while Antibonding Molecular Orbitals (ABOs) result from destructive interference and can potentially weaken the bonding by introducing regions of low electron density.
Q4. How are electrons distributed in Molecular Orbitals?
Answer. Electrons fill molecular orbitals following the Aufbau principle, Pauli exclusion principle, and Hund’s rule. Lower-energy orbitals are filled first, with electrons having opposite spins.
Q5. Can Molecular Orbital Theory be applied to all molecules?
Answer. MO Theory is a powerful tool for explaining the electronic structure of a wide range of molecules, but its accuracy may vary depending on the complexity of the molecule and the degree of interaction between atomic orbitals.

Download Question Bank


Post a Comment