The measurement of changes in internal energy (∆U) and enthalpy (∆H) constitutes a pivotal aspect of thermodynamics, shedding light on fundamental energy transformations within systems. As dynamic fields like chemistry and physics delve into the intricacies of matter and energy, understanding how to quantify alterations in internal energy and enthalpy is paramount. This exploration involves sophisticated methodologies such as calorimetry, offering a glimpse into the heat exchanges that accompany diverse processes. In this brief, we embark on a journey to unravel the significance and methods underlying the measurement of ∆U and ∆H, recognizing their indispensable roles in deciphering the behavior of energy in dynamic systems.
Measuring ∆U and ∆H in Thermodynamics
Understanding the intricacies of energy changes within systems is pivotal in thermodynamics. Measuring changes in internal energy (∆U) and enthalpy (∆H) plays a crucial role in unraveling these complexities. Employing methods like calorimetry, scientists and engineers gain insights into the heat exchanges that accompany various processes. ∆U, representing internal energy changes, is often measured through calorimetric techniques, while ∆H, the change in enthalpy, is determined through direct measurements or applications of Hess's Law. This exploration into the measurement of ∆U and ∆H is fundamental, providing valuable tools for comprehending and optimizing energy transformations in diverse scientific and industrial applications.\
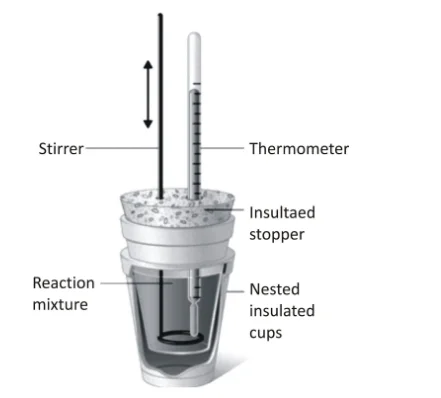
Internal Energy (∆U) Measurement:
Internal energy (∆U) measurement is a fundamental aspect of thermodynamics, providing insights into the energy changes occurring within a system. Internal energy represents the sum of particles' kinetic and potential energies within a system. The change in internal energy, denoted as ∆U, is often measured through the following methods:
-
Calorimetry: Calorimetry is a widely used technique for measuring ∆U. In a calorimeter, the system undergoes a process, and the heat evolved or absorbed is measured. The principle of conservation of energy is applied to relate the heat exchanged to the change in internal energy.
-
Heat Capacity: The heat capacity of a system, denoted as C, is defined as the amount of heat required to raise its temperature by one degree Celsius. The change in internal energy (∆U) is related to heat (Q) and heat capacity (C) through the equation ∆U = Q – P∆V, where P is pressure and ∆V is the volume change.
-
Adiabatic Processes: In certain cases, where a process occurs without heat exchange with the surroundings (adiabatic process), the change in internal energy (∆U) can be determined from the work done on or by the system.
Accurate measurement of internal energy changes is essential for understanding and predicting the behavior of systems undergoing various thermodynamic processes. These measurements are crucial in chemistry, physics, and engineering, providing the foundation for optimizing processes and designing efficient energy systems.
Enthalpy Change (∆H) Measurement:
Enthalpy change (∆H) measurement is a vital aspect of thermodynamics, particularly in the study of chemical reactions and processes occurring at constant pressure. Enthalpy is a state function that includes the internal energy of a system and the product of its pressure and volume. The change in enthalpy, ∆H, is often measured through various methods:
-
Heat of Reaction Measurements: In chemical reactions occurring at constant pressure, the heat evolved or absorbed is measured directly. This heat of reaction is equal to the change in enthalpy (∆H) for the system.
-
Calorimetry: Similar to measuring ∆U, calorimetry is widely employed to measure ∆H. By using a calorimeter, the heat exchanged during a process is determined, providing valuable information about the enthalpy change.
-
Hess's Law: When direct measurements are challenging, Hess's Law is applied. This principle states that the total enthalpy change for a reaction is the same, regardless of the number of steps taken to achieve the final state. By combining known reactions, the ∆H for an overall reaction can be calculated.
Accurate measurement of ∆H is essential in understanding the energy changes associated with chemical reactions and other thermodynamic processes. These measurements have significant implications in fields such as chemistry, biochemistry, and material science, where optimizing reactions and understanding energy transformations are critical.

Download Chemistry Notes
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. Why is measuring internal energy (∆U) important in thermodynamics?
Answer. Measuring ∆U is crucial as it provides insights into the energy changes within a system, aiding in the understanding and prediction of thermodynamic processes. This information is fundamental in optimizing processes and designing efficient energy systems.
Q2. How is internal energy (∆U) measured using calorimetry?
Answer. Calorimetry involves measuring the heat exchanged during a process. In the context of ∆U, the heat transferred is related to the change in internal energy using the principle of conservation of energy, where ∆U equals the heat exchanged minus the work done by the system.
Q3. What is the significance of enthalpy change (∆H) measurements in chemical reactions?
Answer. ∆H measurements in chemical reactions are crucial for understanding the heat changes associated with reactions at constant pressure. This information is vital in fields like chemistry and biochemistry, helping researchers optimize reaction conditions and assess the feasibility of various processes.
Q4. Can Hess's Law be applied to any chemical reaction?
Answer. Yes, Hess's Law can be applied to any chemical reaction. It states that the total enthalpy change for a reaction is the same, regardless of the number of steps taken to achieve the final state. This principle is especially useful when direct measurements of ∆H are challenging.
Q5. How is ∆H measured in calorimetry?
Answer. In calorimetry, ∆H is measured by determining the heat exchanged during a process at constant pressure. The measured heat is equivalent to the change in enthalpy for the system. This method is particularly useful for reactions occurring in open systems where pressure is constant.

Download Question Bank


Post a Comment