A substance that experiences a chemical reaction is said to have chemical reactivity. An element’s ability to displace another element in a single displacement reaction may be ascertained using the reactivity series of elements. It explains how reactive metals are to acids and water, as well as how they may combine to produce compounds.
Metals and non-metals are the two main groups of elements in the periodic table. Irrespective of how reactive they are, these elements are arranged in the table based on their atomic numbers. On the other hand, these components are arranged in a reactivity series in a declining sequence of reactivity.
In this post, we’ll learn about the reactivity series of several elements, including metals and non-metals, as well as their characteristics. We’ll also discuss the significance of reactivity series and reactivity series mnemonics.
Reactivity Series
The arrangement of metals in decreasing order of their reactivity is called the reactivity series, or activity series. As a result, in the reactivity series chart, the metal with the lowest reactivity is positioned at the bottom. On the other hand, the metal at the top of the list has the highest reactivity.
Comparably, we organize non-metals in the decreasing order of their chemical activity to form the reactivity series of non-metals.
Reactivity Series of Elements
The following tips cover the reactivity series of elements, including metals and non-metals:
The periodic table’s components frequently interact with one another to create new compounds. Their atoms’ ability to share, gain, or lose electrons explains this.
Metals: Metals have a constant tendency to lose electrons to create cations, or positive ions, such as Na+, Mg+, etc. A metal is considered highly reactive if, at normal temperature, the atom easily loses electrons.
Non-metals: Generally, non-metals acquire or distribute electrons to generate negative ions known as anions, such as Cl-, SO2−, etc. A non-metal is considered more reactive if its atoms pick up electrons more easily, and vice versa.

Download Mathematics notes
Reactivity Series of Metals

The arrangement of metals in decreasing order of reactivity is known as the reactivity series, or activity series. Here, a few key topics have been covered:
1. Extremely electropositive metals are those at the top of the reactivity scale. Metals become less electro positivity as one moves down the reactivity series.
2. As one moves down the metal reactivity series, the elements’ electropositivity also decreases.
3. When dilute H2SO4 or dilute HCl reacts with any metal in the activity series that is above hydrogen, H2 gas is released.
4. As we move down the series, the metals’ capacity to reduce gets less strong.
5. Higher ranked metals on the reactivity series have the power to push out lower ranked metals from their salt solutions.
6. Higher ranking metals require more energy to separate from ores and other substances.
Reactivity Series of Non-Metals
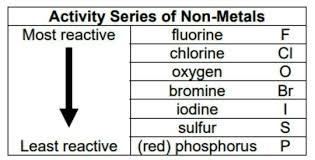
Non-metals can be grouped according to how reactive they are, just like metals. Higher active nonmetals push out lower active non-metals from a molecule during displacement reactions. A non-metal’s ability to transfer electrons to a solution state and create positive ions determines how reactive it is. A non-metal is more active and ranks higher in the reactivity series the more readily it acquires electrons.
.webp)
Download Question Bank
Properties of Reactivity Series
The displacement reaction and the interaction between metals and water are aided by the reactivity series. A more reactive metal pushes a less reactive metal out of its salt in displacement reactions.
The following are a few characteristics of the reactivity series:
1. The reactivity series provides information on how reactive metals are with water.
2. We can determine which metal will displace the other metal using the reactivity series.
3. Elements are arranged in a reactivity series in declining order of reactivity.
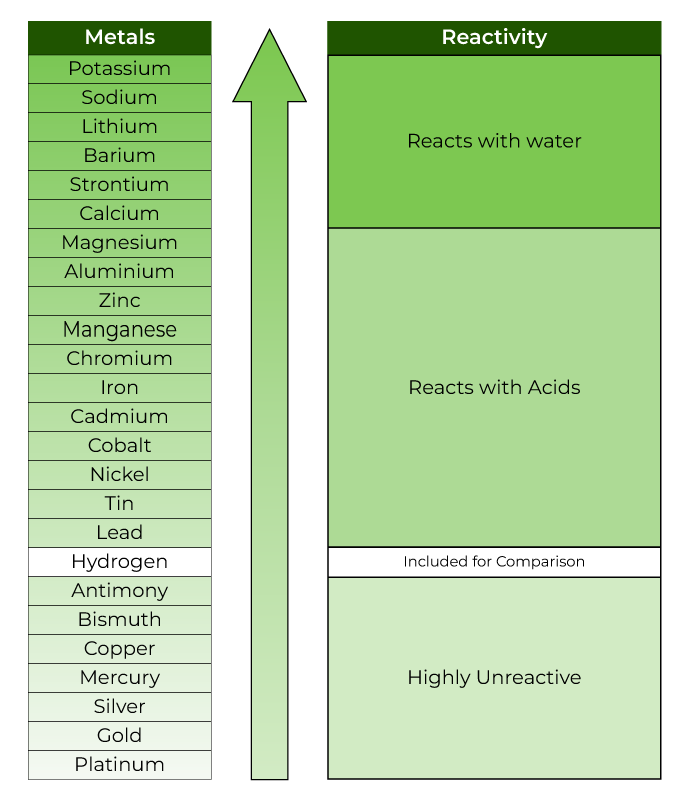
Reactivity Series in Tabular Form
The typical shape of the reactivity series has been seen. The following table displays the reactivity series in tabular form along with ions:
Importance of Reactivity Series
The displacement reaction and the reaction between metals and water are two significant applications for the reactivity series. A more reactive metal pushes a less reactive metal out of its salt in displacement reactions. We can determine which metal will displace the other metal using the reactivity series.
These are a few examples of how metals and water react chemically:
Salt (potassium) and chilly water:
As a result, Cold Potassium Water Hydrogen and Potassium Hydroxide
Ca (magnesium) in cold water:
End Product: Cold Calcium Water Hydrogen and Calcium Hydroxide
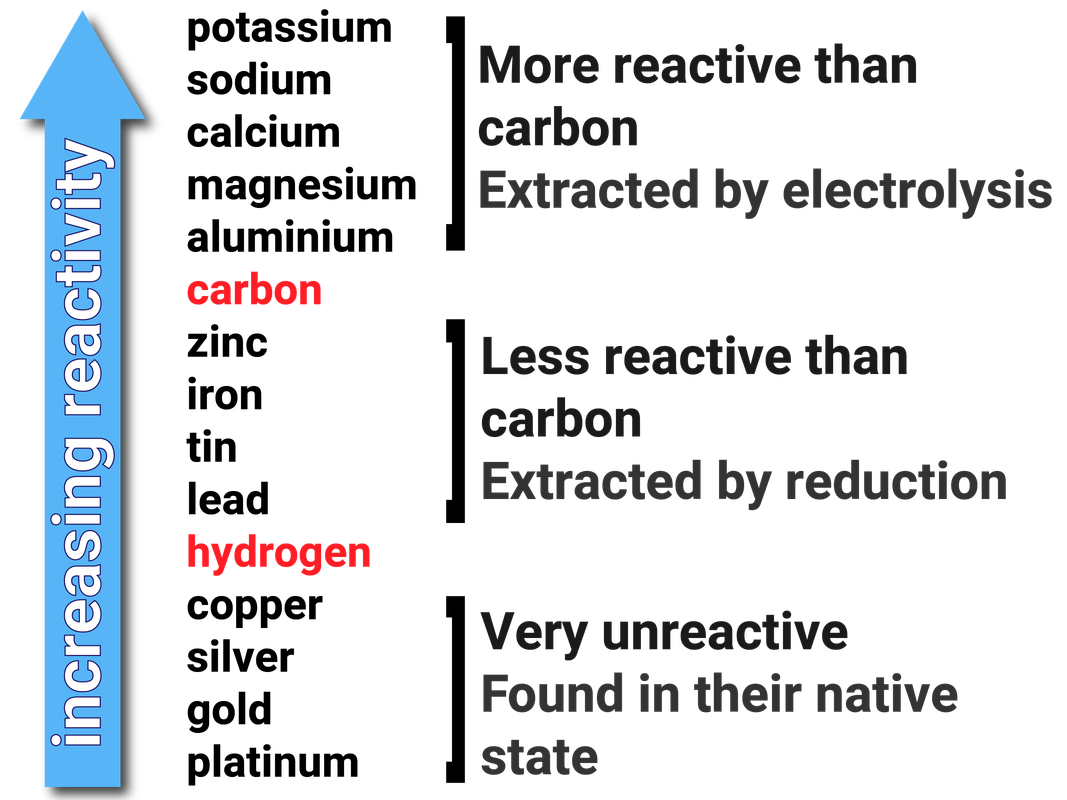
Reactivity Series Mnemonics
There is an easy way to commit the reactivity series to memory. To fully commit the sequence to memory, refer to this line:
CBSE Class 10 NCERT Science Topics for a Strong Foundation
List of CBSE Class 10 Science Chapter
List of CBSE Class 10 Mathmetics Chapter
FAQs
Que1. What is the importance of reactivity series?
Ans: grasp the behavior of metals in different chemical reactions requires a grasp of the reactivity series. It aids in forecasting the possibility of displacement reactions as well as the metals’ relative acid reactivity. In disciplines like chemistry, environmental science, and metallurgy, this knowledge is essential for forecasting reactions and creating effective process designs.
Que2. What is metal reactivity?
Ans: materials such as oxygen or acids, causing compounds to develop or corrosion to occur. Different metals have different levels of reactivity; some, like alkali metals, are quite reactive, while other metals, like noble metals, are more inert.
Que3. Which are the most reactive metals in the reactivity series?
Ans. The most reactive metals in the reactivity series are potassium, sodium, and lithium.
Que4. Name some of the least reactive metals in the reactivity series.
Ans. Gold, silver, and platinum are among the least reactive metals in the reactivity series.
Que5. What is a displacement reaction?
Ans. When a more reactive element pushes out a less reactive element from its compound, it’s called a displacement reaction.


Post a Comment