Hybridization is a captivating concept in the realm of genetics and botany, unlocking the potential for diverse and resilient organisms. It involves the interbreeding of distinct species or varieties, blending their genetic traits to create a hybrid with unique characteristics. This phenomenon has profound implications in agriculture, where hybrid crops often exhibit enhanced vigor and productivity. Additionally, hybridization plays a pivotal role in the evolution of species, fostering adaptability and genetic diversity. In this exploration of hybridization, we delve into its mechanisms, applications, and the transformative impact it has on both natural ecosystems and human endeavors.
Unveiling the Intricacies and Impact of Genetic Hybridization
What is Hybridization?
Hybridization is a molecular biology and genetics concept involving the combination of genetic material from different species or varieties to create a hybrid organism. In chemistry, specifically in atomic orbitals, hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals, influencing the structure and properties of molecules. This process occurs to achieve optimal bonding arrangements and is commonly observed in organic chemistry. Hybridization plays a crucial role in both biological evolution, fostering diversity, and chemical reactivity, influencing the shape and stability of molecules.
Rules for Observing the Type of Hybridisation
- Count the Regions of Electron Density:
- Determine the number of sigma bonds (single, double, and triple) and lone pairs around a central atom.
- Each sigma bond or lone pair counts as one region of electron density.
- Use the Number of Regions to Determine Hybridization:
- 2 regions: sp hybridization
- 3 regions: sp2 hybridization
- 4 regions: sp3 hybridization
- 5 regions: sp3d hybridization
- 6 regions: sp3d2 hybridization
- Consider Double and Triple Bonds:
- Each double bond (one sigma bond and one pi bond) counts as one region.
- Each triple bond (one sigma bond and two pi bonds) counts as one region.
- Take Note of Lone Pairs:
- Lone pairs also contribute to the total count of regions of electron density.
- Observe Molecular Geometry:
- The molecular geometry (shape) is determined by the number of bonding and lone pairs.
- Check for Unusual Hybridizations:
- Some molecules may have hybridizations that deviate from the typical patterns, especially in cases of expanded octets or unusual bonding situations.
Types of Hybridisation
Hybridization is a concept in chemistry that involves the combination of atomic orbitals to form hybrid orbitals, influencing the shape and properties of molecules. Here are the common types of hybridization:
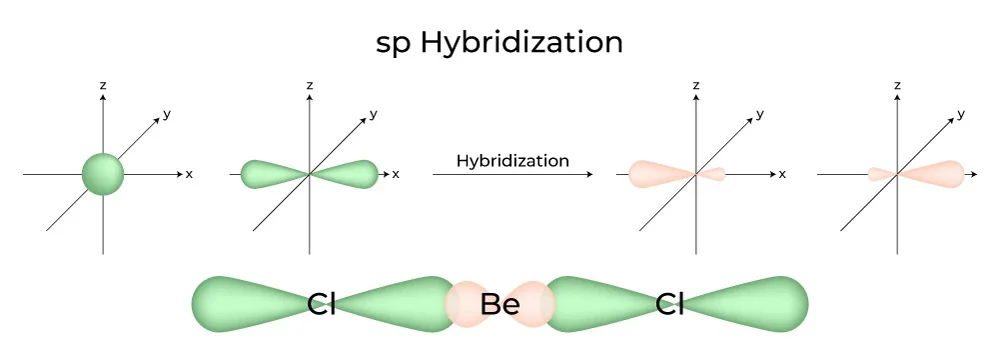
- sp Hybridization:
- Example: Linear molecules like BeH2 and acetylene (C2H2).
- Hybrid orbitals result from the combination of one s and one p orbital.
- sp2 Hybridization:
- Example: Trigonal planar molecules like BF3 and ethene (C2H4).
- Hybrid orbitals form from one s and two p orbitals.
- sp3 Hybridization:
- Example: Tetrahedral molecules like CH4 and ethane (C2H6).
- Hybrid orbitals arise from the combination of one s and three p orbitals.
- sp3d Hybridization:
- Example: Trigonal bipyramidal molecules like PCl5 and SF4.
- Hybrid orbitals result from the combination of one s, three p, and one d orbital.
- sp3d2 Hybridization:
- Example: Octahedral molecules like SF6 and PCl6–.
- Hybrid orbitals form from one s, three p, and two d orbitals.
These hybridization types account for the geometry and bonding characteristics of molecules, providing a framework for understanding the arrangement of atoms in space.

Download Chemistry Notes
Studying the Formation of Various Molecules
- Methane (CH4):
- Formation involves four equivalent C-H σ bonds through the interaction of C-sp3 with H-1s orbitals, resulting in a stable tetrahedral structure.
- Ethane (C2H6):
- Six C-H σ bonds form through C-sp3 and H-1s orbital interactions, while a single C-C σ bond is created by the interaction of C-sp3 orbitals, contributing to a stable molecular structure.
- NH3 Molecule Formation:
- Nitrogen in NH3 is sp3-hybridized, with three 1s-orbitals overlapping three sp3 hybrid orbitals. The resultant NH3 molecule exhibits a bond angle slightly less than the ideal 109.50 due to the presence of one occupied sp3-hybrid orbital.
- C2H4 and C2H2 Molecule Formation:
- C2H4 involves sp2-hybridized carbon atoms, with one 2p-orbital remaining unhybridized to form a π-bond. The sp2-hybrid orbitals contribute to σ-bonds, defining the molecular structure. C2H2 exhibits a triple bond formed by the interaction of two sp-hybridized carbon atoms.
- NH3 and H2O Molecule Formation by sp3 Hybridization:
- In the H2O molecule, oxygen is sp3-hybridized, leading to a bond angle of 105.50 due to two occupied orbitals. The sp3 hybridization contributes to the distinctive geometry of water molecules.
Delving into these molecular formations provides insights into the hybridization, bonding, and geometrical arrangements crucial to understanding the diverse world of chemical structures.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What is hybridization in chemistry?
Answer. Hybridization is a concept in chemistry where atomic orbitals combine to form hybrid orbitals, influencing the shape and bonding of molecules.
Q2. Why does hybridization occur?
Answer. Hybridization occurs to maximize the overlap of atomic orbitals and create more stable molecular structures.
Q3. What are the common types of hybridization?
Answer. Common types include sp, sp2, sp3, sp3d, and sp3d2, each characterized by a specific combination of s and p orbitals.
Q4. Can atoms with different hybridizations bond?
Answer. Yes, atoms with different hybridizations can form bonds, and the resulting molecule may exhibit a hybridization that is a combination of the individual hybridizations.
Q5. What is the significance of sp2 hybridization?
Answer. Molecules with sp2 hybridization, such as BF3 and ethene (C2H4), have trigonal planar geometries, influencing their chemical and physical properties.
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.

Download Question Bank


Post a Comment