The Complete IB Chemistry Syllabus: SL & HL
IB chemistry is little hard. If you are going through this syllabus, I suppose you are interested in taking this course or you are presently joined with this course.
In this article, I am going to discuss every topic covered in IB Chemistry Standard level and IB chemistry higher level along with the number of hours dedicated to each topic.
Both IB chemistry SL & HL core consist of same requirement that consists of a total of 95 hours.
IB DP Chemistry Study Guide

Both classes SL and HL will cover the same 11 topics in the order listed below along with the same subtopics and dedicated hours listed below:
Topic 1: Stoichiometric Relationships – SL & HL combined for 13.5 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Introduction to the particulate nature of matter and chemical change
|
1.1
|
-
Atoms of different elements combine in fixed ratios to form compounds, which have different properties from their component elements.
-
Mixtures contain more than one element and/or compound that are not chemically bonded together and so retain their individual properties.
-
Mixtures are either homogeneous or heterogeneous.
|
|
The mole concept
|
1.2
|
-
The mole is a fixed number of particles and refers to the amount, n, of substance.
-
Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr).
-
Molar mass (M) has the units g mol-1.
-
The empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atoms present in a molecule respectively.
|
|
Reacting masses and volumes
|
1.3
|
-
Reactants can be either limiting or excess.
-
The experimental yield can be different from the theoretical yield.
-
Avogadro’s law enables the mole ratio of reacting gases to be determined from volumes of the gases.
-
The molar volume of an ideal gas is a constant at specified temperature and pressure.
-
The molar concentration of a solution is determined by the amount of solute and the volume of solution.
-
A standard solution is one of known concentration.
|
Topic 2: Atomic Structure – SL & HL combined for 6 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
The nuclear atom
|
2.1
|
-
Atoms contain a positively charged dense nucleus composed of protons and neutrons (nucleons).
-
Negatively charged electrons occupy the space outside the nucleus.
-
The mass spectrometer is used to determine the relative atomic mass of an element from its isotopic composition."
|
|
Electron configuration
|
2.2
|
-
Emission spectra are produced when photons are emitted from atoms as excited electrons return to a lower energy level.
-
The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
-
The main energy level or shell is given an integer number, n, and can hold a maximum number of electrons, 2n2.
-
A more detailed model of the atom describes the division of the main energy level into s, p, d and f sub-levels of successively higher energies.
-
Sub-levels contain a fixed number of orbitals, regions of space where there is a high probability of finding an electron.
-
Each orbital has a defined energy state for a given electronic configuration and chemical environment and can hold two electrons of opposite spin.
|
Get Free IB Trial Session Download IB eBook

Topic 3: Periodicity – SL & HL combined for 6 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Periodic table
|
3.1
|
-
The periodic table is arranged into four blocks associated with the four sub- levels—s, p, d, and f.
-
The periodic table consists of groups (vertical columns) and periods (horizontal rows).
-
The period number (n) is the outer energy level that is occupied by electrons.
-
The number of the principal energy level and the number of the valence electrons in an atom can be deduced from its position on the periodic table.
-
The periodic table shows the positions of metals, non-metals and metalloids.
|
|
Periodic trends
|
3.2
|
-
Vertical and horizontal trends in the periodic table exist for atomic radius, ionic radius, ionization energy, electron affinity and electronegativity.
-
Trends in metallic and non-metallic behaviour are due to the trends above.
-
Oxides change from basic through amphoteric to acidic across a period.
|
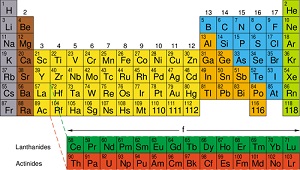
Topic 4: Chemical Bonding and Structure – SL & HL combined for 13.5 hours
| Subtopic |
Subtopic number |
Interpretations |
| Ionic bonding and structure |
4.1 |
- Positive ions (cations) form by metals losing valence electrons.
- Negative ions (anions) form by non-metals gaining electrons.
- The number of electrons lost or gained is determined by the electron configuration of the atom.
- The ionic bond is due to electrostatic attraction between oppositely charged ions.
- Under normal conditions, ionic compounds are usually solids with lattice structures.
|
| Covalent bonding |
4.2 |
- A covalent bond is formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei.
- Single, double and triple covalent bonds involve one, two and three shared pairs of electrons respectively.
- Bond length decreases and bond strength increases as the number of shared electrons increases.
- Bond polarity results from the difference in electronegativities of the bonded atoms.
|
| Covalent structures |
4.3 |
- Lewis (electron dot) structures show all the valence electrons in a covalently bonded species.
- The “octet rule” refers to the tendency of atoms to gain a valence shell with a total of 8 electrons.
- Some atoms, like Be and B, might form stable compounds with incomplete octets of electrons.
- Resonance structures occur when there is more than one possible position for a double bond in a molecule.
- Shapes of species are determined by the repulsion of electron pairs according to VSEPR theory.
- Carbon and silicon form giant covalent/network covalent structures.
|
| Intermolecular forces |
4.4 |
- Intermolecular forces include London (dispersion) forces, dipole-dipole forces and hydrogen bonding.
- The relative strengths of these interactions are London (dispersion) forces < dipole-dipole forces < hydrogen bonds.
|
| Metallic bonding |
4.5 |
- A metallic bond is the electrostatic attraction between a lattice of positive ions and delocalized electrons.
- The strength of a metallic bond depends on the charge of the ions and the radius of the metal ion.
- Alloys usually contain more than one metal and have enhanced properties.
|

IB DP Chemistry Study Guide
Topic 5: Energetics / Thermochemistry – SL & HL combined for 9 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Measuring energy changes
|
5.1
|
-
Heat is a form of energy.
-
Temperature is a measure of the average kinetic energy of the particles.
-
Total energy is conserved in chemical reactions.
-
Chemical reactions that involve transfer of heat between the system and the surroundings are described as endothermic or exothermic.
-
The enthalpy change (∆H) for chemical reactions is indicated in kJ mol-1.
-
∆H values are usually expressed under standard conditions, given by ∆H°, including standard states.
|
|
Hess’s Law
|
5.2
|
|
|
Bond enthalpies
|
5.3
|
|
Topic 6: Chemical Kinetics – SL & HL combined for 7 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Collision theory and rates of reaction
|
6.1
|
-
Species react as a result of collisions of sufficient energy and proper orientation.
-
The rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time.
-
Concentration changes in a reaction can be followed indirectly by monitoring changes in mass, volume and colour.
-
Activation energy (Ea) is the minimum energy that colliding molecules need in order to have successful collisions leading to a reaction.
-
By decreasing Ea, a catalyst increases the rate of a chemical reaction, without itself being permanently chemically changed.
|
Topic 7: Equilibrium – SL & HL combined for 4.5 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Equilibrium
|
7.1
|
-
A state of equilibrium is reached in a closed system when the rates of the forward and reverse reactions are equal.
-
The equilibrium law describes how the equilibrium constant (Kc) can be determined for a particular chemical reaction.
-
The magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent.
-
The reaction quotient (Q) measures the relative amount of products and reactants present during a reaction at a particular point in time. Q is the equilibrium expression with non-equilibrium concentrations. The position of the equilibrium changes with changes in concentration, pressure, and temperature.
-
A catalyst has no effect on the position of equilibrium or the equilibrium constant.
|
Topic 8: Acids and Bases – SL & HL combined for 6.5 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Theories of acids and bases
|
8.1
|
-
A Brønsted–Lowry acid is a proton/H+ donor and a Brønsted–Lowry base is a proton/H+ acceptor.
-
Amphiprotic species can act as both Brønsted–Lowry acids and bases.
-
A pair of species differing by a single proton is called a conjugate acid-base pair.
|
|
Properties of acids and bases
|
8.2
|
-
Most acids have observable characteristic chemical reactions with reactive metals, metal oxides, metal hydroxides, hydrogen carbonates and carbonates.
-
Salt and water are produced in exothermic neutralization reactions.
|
|
The pH scale
|
8.3
|
-
pH = − log[H+(aq)] and [H+] = 10−pH.
-
A change of one pH unit represents a 10-fold change in the hydrogen ion concentration [𝐻𝐻+].
-
pH values distinguish between acidic, neutral and alkaline solutions.
-
The ionic product constant, 𝐾𝐾𝑤𝑤 = [H+][OH−] = 10−14 at 298 K.
|
|
Strong and weak acids and bases
|
8.4
|
-
Strong and weak acids and bases differ in the extent of ionization.
-
Strong acids and bases of equal concentrations have higher conductivities than weak acids and bases.
-
A strong acid is a good proton donor and has a weak conjugate base.
-
A strong base is a good proton acceptor and has a weak conjugate acid.
|
|
Acid deposition
|
8.5
|
-
Rain is naturally acidic because of dissolved CO2 and has a pH of 5.6. Acid deposition has a pH below 5.6.
-
Acid deposition is formed when nitrogen or sulfur oxides dissolve in water to form HNO3, HNO2, H2SO4 and H2SO3.
-
Sources of the oxides of sulfur and nitrogen and the effects of acid deposition should be covered.
|

Topic 9: Redox Processes – SL & HL combined for 8 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Oxidation and reduction
|
9.1
|
-
Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron transfer or change in oxidation number.
-
An oxidizing agent is reduced and a reducing agent is oxidized.
-
Variable oxidation numbers exist for transition metals and for most main-group non-metals.
-
The activity series ranks metals according to the ease with which they undergo oxidation.
-
The Winkler Method can be used to measure biochemical oxygen demand (BOD), used as a measure of the degree of pollution in a water sample.
|
|
Electrochemical cells
|
9.2
|
Voltaic (Galvanic) cells
-
Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
-
Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell.
Electrolytic cells
-
Electrolytic cells convert electrical energy to chemical energy, by bringing about non-spontaneous processes.
-
Oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode) in an electrolytic cell.
|
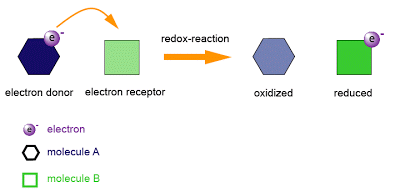
IB DP Chemistry Study Guide
Topic 10: Organic Chemistry – SL & HL combined for 11 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Fundamentals of organic chemistry
|
10.1
|
-
A homologous series is a series of compounds of the same family, with the same general formula, which differ from each other by a common structural unit.
-
Structural formulas can be represented in full and condensed format.
-
Structural isomers are compounds with the same molecular formula but different arrangements of atoms.
-
Functional groups are the reactive parts of molecules.
-
Saturated compounds contain single bonds only and unsaturated compounds contain double or triple bonds.
-
Benzene is an aromatic, unsaturated hydrocarbon.
|
|
Functional group chemistry
|
10.2
|
Alkanes:
Alkenes:
Alcohols:
Halogenoalkanes:
Polymers:
Benzene:
|
Topic 11: Measurement and Data Processing – SL & HL combined for 10 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Uncertainties and errors in measurement and results
|
11.1
|
-
Qualitative data includes all non-numerical information obtained from observations not from measurement.
-
Quantitative data are obtained from measurements, and are always associated with random errors/uncertainties, determined by the apparatus, and by human limitations such as reaction times.
-
Propagation of random errors in data processing shows the impact of the uncertainties on the final result.
-
Experimental design and procedure usually lead to systematic errors in measurement, which cause a deviation in a particular direction.
-
Repeat trials and measurements will reduce random errors but not systematic errors.
|
|
Graphical techniques
|
11.2
|
-
Graphical techniques are an effective means of communicating the effect of an independent variable on a dependent variable, and can lead to determination of physical quantities.
-
Sketched graphs have labeled but unscaled axes, and are used to show qualitative trends, such as variables that are proportional or inversely proportional.
-
Drawn graphs have labeled and scaled axes, and are used in quantitative measurements.
|
|
Spectroscopic identification of organic compounds
|
11.3
|
-
The degree of unsaturation or index of hydrogen deficiency (IHD) can be used to determine from a molecular formula the number of rings or multiple bonds in a molecule.
-
Mass spectrometry (MS), proton nuclear magnetic resonance spectroscopy (1H NMR) and infrared spectroscopy (IR) are techniques that can be used to help identify compounds and to determine their structure.
|
Additional Higher Level Topics
These classes are assigned for Higher Level students only with a total of 60 hours.
Topic 12: Atomic Structure – HL only for 2 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Electrons in atoms
(HL ONLY)
|
12.1
|
-
In an emission spectrum, the limit of convergence at higher frequency corresponds to the first ionization energy.
-
Trends in first ionization energy across periods account for the existence of main energy levels and sub-levels in atoms.
-
Successive ionization energy data for an element give information that shows relations to electron configurations.
|
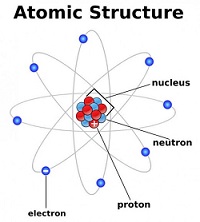
Topic 13: The Periodic Table-the Transition Metals -HL only for 4 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
First-row d-block elements
(HL ONLY)
|
13.1
|
-
Transition elements have variable oxidation states, form complex ions with ligands, have coloured compounds, and display catalytic and magnetic properties.
-
Zn is not considered to be a transition element as it does not form ions with incomplete d-orbitals.
-
Transition elements show an oxidation state of +2 when the s-electrons are removed.
|
|
Coloured complexes
(HL ONLY)
|
13.2
|
-
The d sub-level splits into two sets of orbitals of different energy in a complex ion.
-
Complexes of d-block elements are coloured, as light is absorbed when an electron is excited between the d-orbitals.
-
The colour absorbed is complementary to the colour observed.
|
Topic 14: Chemical Bonding and Structure – HL only for 7 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Further aspects of covalent bonding and structure
(HL ONLY)
|
14.1
|
-
Covalent bonds result from the overlap of atomic orbitals. A sigma bond (σ) is formed by the direct head-on/end-to-end overlap of atomic orbitals, resulting in electron density concentrated between the nuclei of the bonding atoms. A pi bond (π) is formed by the sideways overlap of atomic orbitals, resulting in electron density above and below the plane of the nuclei of the bonding atoms.
-
Formal charge (FC) can be used to decide which Lewis (electron dot) structure is preferred from several. The FC is the charge an atom would have if all atoms in the molecule had the same electronegativity. FC = (Number of valence electrons)-½(Number of bonding electrons)-(Number of non-bonding electrons). The Lewis (electron dot) structure with the atoms having FC values closest to zero is preferred.
-
Exceptions to the octet rule include some species having incomplete octets and expanded octets.
-
Delocalization involves electrons that are shared by/between all atoms in a molecule or ion as opposed to being localized between a pair of atoms.
-
Resonance involves using two or more Lewis (electron dot) structures to represent a particular molecule or ion. A resonance structure is one of two or more alternative Lewis (electron dot) structures for a molecule or ion that cannot be described fully with one Lewis (electron dot) structure alone.
|
|
Hybridization
(HL ONLY)
|
14.2
|
|
Topic 15: Energetics / Thermochemistry – HL only for 7 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Energy cycles
(HL ONLY)
|
15.1
|
-
Representative equations (eg M+(g) M+(aq)) can be used for enthalpy/energy of hydration, ionization, atomization, electron affinity, lattice, covalent bond and solution.
-
Enthalpy of solution, hydration enthalpy and lattice enthalpy are related in an energy cycle.
|
|
Entropy and spontaneity
(HL ONLY)
|
15.2
|
-
Entropy (S) refers to the distribution of available energy among the particles. The more ways the energy can be distributed the higher the entropy.
-
Gibbs free energy (G) relates the energy that can be obtained from a chemical reaction to the change in enthalpy (ΔH), change in entropy (ΔS), and absolute temperature (T).
-
Entropy of gas>liquid>solid under same conditions.
|
Topic 16: Chemical Kinetics – HL only for 6 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Rate expression and reaction mechanism
(HL ONLY)
|
16.1
|
-
Reactions may occur by more than one step and the slowest step determines the rate of reaction (rate determining step/RDS).
-
The molecularity of an elementary step is the number of reactant particles taking part in that step.
-
The order of a reaction can be either integer or fractional in nature. The order of a reaction can describe, with respect to a reactant, the number of particles taking part in the rate-determining step.
-
Rate equations can only be determined experimentally.
-
The value of the rate constant (k) is affected by temperature and its units are determined from the overall order of the reaction.
-
Catalysts alter a reaction mechanism, introducing a step with lower activation energy.
|
|
Activation energy
(HL ONLY)
|
16.2
|
-
The Arrhenius equation uses the temperature dependence of the rate constant to determine the activation energy.
-
A graph of 1/T against ln k is a linear plot with gradient – Ea / R and intercept, lnA.
-
The frequency factor (or pre-exponential factor) (A) takes into account the frequency of collisions with proper orientations.
|
Topic 17: Equilibrium – HL only for 4 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
The equilibrium law
(HL ONLY)
|
17.1
|
-
Le Châtelier’s principle for changes in concentration can be explained by the equilibrium law.
-
The position of equilibrium corresponds to a maximum value of entropy and a minimum in the value of the Gibbs free energy.
-
The Gibbs free energy change of a reaction and the equilibrium constant can both be used to measure the position of an equilibrium reaction and are related by the equation, ∆G° = −RT ln(𝐾)
|
Topic 18: Acids and Bases – HL Only for 10 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Lewis acids and bases
(HL ONLY)
|
18.1
|
-
A Lewis acid is a lone pair acceptor and a Lewis base is a lone pair donor.
-
When a Lewis base reacts with a Lewis acid a coordinate bond is formed.
-
A nucleophile is a Lewis base and an electrophile is a Lewis acid.
|
|
Calculations involving acids and bases (HL ONLY)
|
18.2
|
-
The expression for the dissociation constant of a weak acid (Ka) and a weak base (Kb).
-
For a conjugate acid base pair, Ka × Kb = Kw.
-
The relationship between Ka and pKa is (pKa = -log Ka), and between Kb and pKb is (pKb = -log Kb).
|
|
pH curves
(HL ONLY)
|
18.3
|
-
The characteristics of the pH curves produced by the different combinations of strong and weak acids and bases.
-
An acid–base indicator is a weak acid or a weak base where the components of the conjugate acid–base pair have different colours.
-
The relationship between the pH range of an acid–base indicator, which is a weak acid, and its pKa value.
-
The buffer region on the pH curve represents the region where small additions of acid or base result in little or no change in pH.
-
The composition and action of a buffer solution.
|
Topic 19: Redox Processes – HL only for 6 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Electrochemical cells (HL ONLY)
|
19.1
|
-
A voltaic cell generates an electromotive force (EMF) resulting in the movement of electrons from the anode (negative electrode) to the cathode (positive electrode) via the external circuit. The EMF is termed the cell potential (Eº).
-
The standard hydrogen electrode (SHE) consists of an inert platinum electrode in contact with 1 mol dm-3 hydrogen ion and hydrogen gas at 100 kPa and 298 K. The standard electrode potential (Eº) is the potential (voltage) of the reduction half-equation under standard conditions measured relative to the SHE. Solute concentration is 1 mol dm-3 or 100 kPa for gases. Eº of the SHE is 0 V.
-
When aqueous solutions are electrolysed, water can be oxidized to oxygen at the anode and reduced to hydrogen at the cathode
-
ΔGº = -nFEº . When Eº is positive, ΔGº is negative indicative of a spontaneous process. When Eº is negative, ΔGº is positive indicative of a non-spontaneous process. When Eº is 0, then ΔGº is 0.
-
Current, duration of electrolysis and charge on the ion affect the amount of product formed at the electrodes during electrolysis.
-
Electroplating involves the electrolytic coating of an object with a metallic thin layer.
|
Topic 20: Organic Chemistry – HL only for 12 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Types of organic reactions
(HL ONLY)
|
20.1
|
Nucleophilic Substitution Reactions:
-
SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular substitution reaction. SN1 involves a carbocation intermediate. SN2 involves a concerted reaction with a transition state.
-
For tertiary halogenoalkanes the predominant mechanism is SN1 and for primary halogenoalkanes it is SN2. Both mechanisms occur for secondary halogenoalkanes.
-
The rate determining step (slow step) in an SN1 reaction depends only on the concentration of the halogenoalkane, rate = k[halogenoalkane]. For SN2, rate = k[halogenoalkane][nucleophile]. SN2 is stereospecific with an inversion of configuration at the carbon.
-
SN2 reactions are best conducted using aprotic, non-polar solvents and SN1 reactions are best conducted using protic, polar solvents.
Electrophilic Addition Reactions:
-
An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids.
-
Markovnikov’s rule can be applied to predict the major product in electrophilic addition reactions of unsymmetrical alkenes with hydrogen halides and interhalogens. The formation of the major product can be explained in terms of the relative stability of possible carbocations in the reaction mechanism.
Electrophilic Substitution Reactions:
Reduction Reactions:
|
|
Synthetic routes
(HL ONLY)
|
20.2
|
-
The synthesis of an organic compound stems from a readily available starting material via a series of discrete steps. Functional group interconversions are the basis of such synthetic routes.
-
Retro-synthesis of organic compounds.
|
|
Stereoisomerism
(HL ONLY
|
20.3
|
-
Stereoisomers are subdivided into two classes -conformational isomers, which interconvert by rotation about a σ bond and configurational isomers that interconvert only by breaking and reforming a bond. Configurational isomers are further subdivided into cis-trans and E/Z isomers and optical isomers.
-
Cis-trans isomers can occur in alkenes or cycloalkanes (or heteroanalogues) and differ in the positions of atoms (or groups) relative to a reference plane. According to IUPAC, E/Z isomers refer to alkenes of the form R1R2C=CR3R4 (R1 ≠ R2, R3 ≠ R4) where neither R1 nor R2 need be different from R3 or R4.
-
A chiral carbon is a carbon joined to four different atoms or groups.
-
An optically active compound can rotate the plane of polarized light as it passes through a solution of the compound. Optical isomers are enantiomers. Enantiomers are non-superimposeable mirror images of each other. Diastereomers are not mirror images of each other.
-
A racemic mixture (or racemate) is a mixture of two enantiomers in equal amounts and is optically inactive.
|
Topic 21: Measurement and Analysis – HL Only for 2 hours
|
Subtopic
|
Subtopic number
|
Interpretations
|
|
Spectroscopic identification of organic compounds (HL ONLY)
|
21.1
|
-
Structural identification of compounds involves several different analytical techniques including IR, 1H NMR and MS.
-
In a high resolution 1H NMR spectrum, single peaks present in low resolution can split into further clusters of peaks.
-
The structural technique of single crystal X-ray crystallography can be used to identify the bond lengths and bond angles of crystalline compounds.
|
Options
In the context of the IB Chemistry class you also cover an additional subject of your choice from the list below( generally you don’t choose, but instead your teacher does). According to the choice you will cover 5-7 topics (15 hours total) for SL and an additional 3-4 topics (25 hours total) for HL.
Option A: Materi


















Post a Comment